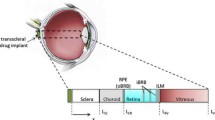
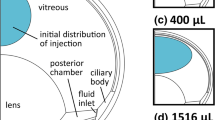
Abstract
Purpose
To determine the outward permeability of retina-choroid-sclera (RCS) layer for different ophthalmic drugs and to develop correlations between drug physicochemical properties and RCS permeability.
Methods
A finite volume model was developed to simulate pharmacokinetics in the eye following drug administration by intravitreal injection. The RCS permeability was determined for 32 compounds by best fitting the drug concentration-time profile obtained by simulation with previously reported experimental data. Multiple linear regression was then used to develop correlations between best fit RCS permeability and drugs physicochemical properties.
Results
The RCS drug permeabilities had values that ranged over 3 × 10−6 m/s. Regression analysis for hydrophilic compounds showed that more than 92% of the variation in permeability values can be explained by correlative models of drug properties that include logarithm of the octanol-water partition coefficient (LogP), protein binding (PB), number of hydrogen bond acceptors (HBA), hydrogen bond donors (HBD), polar surface area (PSA) and dissociation constant (pKa) as independent variables. Regression analysis for lipophilic compounds showed that no significant correlation can be found between just physicochemical properties and RCS permeability.
Conclusion
Using the RCS permeability obtained from this study for different drugs, one can predict pharmacokinetics of intravitreal drug delivery systems such as solid implants or colloidal systems. Furthermore, the developed correlations between RCS permeability and physicochemical properties of drugs are useful in early drug development by predicting RCS permeability and drug concentration in the vitreous without experimental data.




Similar content being viewed by others
Abbreviations
- AUC0-t :
-
area under the concentration-time curve
- AUMC:
-
area under the first moment concentration-time curve
- CFD:
-
computational fluid dynamic
- HBA:
-
hydrogen bond acceptors
- HBD:
-
hydrogen bond donors
- HPLC:
-
high performance liquid chromatography
- KRCS :
-
permeability of retina-choroid-sclera membrane
- LogDC:
-
logarithm of distribution coefficient at pH 7
- LogP:
-
logarithm of the octanol-water partition coefficient of the neutral form
- MRT:
-
mean residence time
- MW:
-
molecular weight
- MLR:
-
multiple linear regression
- PB:
-
protein binding
- pKa:
-
ionization constant in water
- PSA:
-
polar surface area
- RCS:
-
retina-choroid-sclera membrane
References
Geroski DH, Edelhauser HF. Drug delivery for posterior segment eye disease. Invest Ophthalmol Vis Sci. 2000;41(5):961–4.
Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005;57:2010–32.
Chastain JE. General considerations in ocular drug therapy. In: Mitra AK, editor. Drug delivery systems. New York: Marcel Dekker; 2003. p. 59–107.
Maurice DM, Mishima S. Ocular pharmacokinetics. In: Sears ML, editor. Pharmacology of the eye. Berlin: Springer-Verlag; 1984. p. 19–116.
Araie M, Maurice DM. The loss of fluorescein, fluorescein glucuronide and fluorescein isothiocyanate dextran from the vitreous by the anterior and retinal pathways. Exp Eye Res. 1991;52:27–39.
Graham RO, Peyman GA. Intravitreal injection of dexamethasone: treatment of experimentally induced endophthalmitis. Arch Ophthalmol. 1974;92:149–54.
Pitkänen L. Retinal pigment epithelium as a barrier in drug permeation and as a target of non-viral gene delivery, Ph.D. dissertation, University of Kuopio, 2007.
Missel P, Chastain J, Mitra A, Kompella U, Kansara V, Duvvuri S, Amrite A, Cheruvu N. In vitro transport and partitioning of AL-4940, active metabolite of angiostatic agent anecortave acetate, in ocular tissues of the posterior segment. J Ocul Pharmacol Ther. 2010;26(2):137–45.
Hornof M, Toropainen E, Urtti A. Cell culture models of the ocular barriers. Eur J Pharm Biopharm. 2005;60:207–25.
Ohtori A, Tojo K. In vivo/in vitro correlation of intravitreal delivery of drugs with the help of computer simulation. Biol Pharm Bull. 1994;17:283–90.
Friedrich S, Cheng YL, Saville B. Finite element modeling of drug distribution in the vitreous humor of the rabbit eye. Ann Biomed Eng. 1997;25:303–14.
Missel PJ. Hydraulic flow and vascular clearance influences on intravitreal drug delivery. Pharm Res. 2002;19:1636–47.
Missel PJ. Finite and infinitesimal representations of the vasculature: ocular drug clearance by vascular and hydraulic effects. Ann Biomed Eng. 2002;30:1128–39.
Xu J, Heys JJ, Barocas VH, Randolph TW. Permeability and diffusion in vitreous humor: implications for drug delivery. Pharm Res. 2000;17:664–9.
Missel PJ, Horner M, Muralikrishnan R. Simulating dissolution of intravitreal triamcinolone acetonide suspensions in an anatomically accurate rabbit eye model. Pharm Res. 2010;27:1530–46.
Shockley RK, Jay WM, Friberg TR, Aziz AM, Rissing JP, Aziz MZ. Intravitreal ceftriaxone in a rabbit model. Dose- and time-dependent toxic effects and pharmacokinetic analysis. Arch Ophthalmol. 1984;102:1236–8.
Mälkiä A, Murtomäki L, Urtti A, Kontturi K. Drug permeation in biomembranes: in vitro and in silico prediction and influence of physicochemical properties. Eur J Pharm Sci. 2004;23:13–47.
Cunha-Vaz JG, Maurice DM. The active transport of fluorescein by the retinal vessels and the retina. J Physiol. 1967;191:467–86.
Ho NFH, Raub TJ, Burtoon PS, Barsutin CL, Adson A, Audus KL, Borchardt RT. Quantitative approaches to delineate passive transport mechanisms in cell culture monolayers. In: Amidon GL, Lee PI, Topp EM, editors. Transport processes in pharmaceutical systems. New York: Marcel Dekker; 1999. p. 219–316.
Sunkara G, Kompella UB. Membrane transport processes in the eye. In: Mitra AK, editor. Ophthalmic drug delivery systems. New York: Marcel Dekker; 2003. p. 14.
Hillery AM, Lloyd AW, Swarbrick J. Drug delivery and targeting for pharmacists and pharmaceutical scientists. Taylor & Francis; 2001. p. 21.
Clark DE. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 1. Prediction of intestinal absorption. J Pharm Sci. 1999;88:807–14.
Van de Waterbeemd H, Smith DA, Beaumont K, Walker DK. Property-based design: optimisation of drug absorption and pharmacokinetics. J Med Chem. 2001;44:1313–33.
Leeds JM, Henry SP, Truong L, Zutshi A, Levin AA, Kornbrust D. Pharmacokinetics of a potential human cytomegalovirus therapeutic, a phosphorothioate oligonucleotide, after intravitreal injection in the rabbit. Drug Metab Dispos. 1997;25:921–6.
Kim H, Csaky KG, Chan CC, Bungay PM, Lutz RJ, Dedrick RL, Yuan P, Rosenberg J, Grillo-Lopez AJ, Wilson WH, Robinson MR. The pharmacokinetics of rituximab following an intravitreal injection. Exp Eye Res. 2006;82:760–6.
Kim EK, Kim HB. Pharmacokinetics of intravitreally injected liposome-encapsulated tobramycin in normal rabbits. Yonsei Med J. 1990;31:308–14.
Blondeau P, Tetrault JP, Papamarkakis C. Diurnal variation of episcleral venouspressure in healthy patients: a pilot study. J Glaucoma. 2001;10:18–24.
Durairaj C, Shah JC, Senapati S, Kompella UB. Prediction of vitreal half-life based on drug physicochemical properties: quantative structure-pharmacokinetic relationships (QSPKR). Pharm Res. 2009;26:1236–60.
Balachandran RK, Barocas VH. Computer modelling of drug delivery to the posterior eye: effect of active transport and loss to choroidal blood flow. Pharm Res. 2008;25:2685–96.
Kaufman PL, Alm A. Adlers’s physiology of the eye. 10th ed. Year book: Mosby; 2003.
Stay MS, Xu J, Randolph TW, Barocas VH. Computer simulation of convective and diffusive transport of controlled release drugs in the vitreous humor. Pharmaceut Res. 2003;20:96–102.
Hughes PM, Krishnamoorthy R, Mitra AK. Vitreous disposition of two acycloguanosine antivirals in the albino and pigmented rabbit models: a novel ocular microdialysis technique. J Ocular Pharmacol Ther. 1996;12:209–24.
Macha S, Mitra AK. Ocular pharmacokinetics of cephalosporins using microdialysis. J Ocular Pharmacol Ther. 2001;17:485–98.
Liu W, Liu QF, Perkins R, Drusano G, Louie A, Madu A, Mian U, Mayers M, Miller MH. Pharmacokinetics of sparfloxacin in the serum and vitreous humor of rabbits: physicochemical properties that regulate penetration of quinolone antimicrobials. Antimicrob Agents Chemother. 1998;42:1417–23.
Unal M, Peyman GA, Liang C, Hegazy H, Molinari LC, Chen J, Brun S, Tarcha PJ. Ocular toxicity of intravitreal clarithromycin. Retina. 1999;19:442–6.
Pearson PA, Jaffe GJ, Martin DF, Cordahi GJ, Grossniklaus H, Schmeisser ET, Ashton P. Evaluation of a delivery system providing long-term release of cyclosporine. Arch Ophthalmol. 1996;114:311–7.
Anand BS, Atluri H, Mitra AK. Validation of an ocular microdialysis technique in rabbits with permanently implanted vitreous probes: systemic and intravitreal pharmacokinetics of fluorescein. Int J Pharm. 2004;281:79–88.
Macha S, Mitra AK. Ocular disposition of ganciclovir and its monoester prodrugs following intravitreal administration using microdialysis. Drug Metab Dispos. 2002;30:670–5.
Solans C, Bregante MA, Garcia MA, Perez S. Ocular penetration of grepafloxacin after intravitreal administration in albino and pigmented rabbits. Chemotherapy. 2004;50:133–7.
Schenk G, Peyman GA. Lincomycin by direct intravitreal injection in the treatment of experimental bacterial endophthalmitis. Albrecht Von Graefes Arch. Klin Exp Ophthalmol. 1974;190:281–91.
Atluri H, Talluri RS, Mitra AK. Functional activity of a large neutral amino acid transporter (LAT) in rabbit retina: a study involving the in vivo retinal uptake and vitreal pharmacokinetics of l-phenyl alanine. Int J Pharm. 2008;347:23–30.
Velez G, Yuan P, Sung C, Tansey G, Reed GF, Chan CC, Nussenblatt RB, Robinson MR. Pharmacokinetics and toxicity of intravitreal chemotherapy for primary intraocular lymphoma. Arch Ophthalmol. 2001;119:1518–24.
Smith MA, Sorenson JA, Smith C, Miller M, Borenstein M. Effects of intravitreal dexamethasone on concentration of intravitreal vancomycin in experimental methicillin-resistant Staphylococcus epidermidis endophthalmitis. Antimicrob Agents Chemother. 1991;35:1298–302.
Shen YC, Wang MY, Wang CY, Tsai TC, Tsai HY, Lee YF, Wei LC. Clearance of intravitreal voriconazole. Invest Ophthalmol Vis Sci. 2007;48:2238–41.
Atluri H, Mitra AK. Disposition of short-chain aliphatic alcohols in rabbit vitreous by ocular microdialysis. Exp Eye Res. 2003;76:315–20.
Dias CS, Mitra AK. Vitreal elimination kinetics of large molecular weight FITC-labeled dextrans in albino rabbits using a novel microsampling technique. J Pharm Sci. 2000;89:572–8.
Koh HJ, Cheng L, Bessho K, Jones TR, Davidson MC, Freeman WR. Intraocular properties of urokinase-derived antiangiogenic A6 peptide in rabbits. J Ocular Pharmacol Ther. 2004;20:439–49.
Cundy KC, Lynch G, Shaw JP, Hitchcock MJ, Lee WA. Distribution and metabolism of intravitreal cidofovir and cyclic HPMPC in rabbits. Curr Eye Res. 1996;15:569–76.
Berthe P, Baudouin C, Garraffo R, Hofmann P, Taburet AM, Lapalus P. Toxicologic and pharmacokinetic analysis of intravitreal injections of foscarnet, either alone or in combination with ganciclovir. Invest Ophthalmol Vis Sci. 1994;35:1038–45.
Peyman GA, May DR, Ericson ES, Apple D. Intraocular injection of gentamicin. Toxic effects of clearance. Arch Ophthalmol. 1974;92:42–7.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors wish to thank NSERC, 20/20 Ophthalmic Material Network of Canada for supporting this project and Dr. M.R. Pishvaie for his assistance.
Author information
Authors and Affiliations
Corresponding author
Appendices
APPENDIX I
CFD Model Construction
Governing Equations
Bulk flow through the vitreous body occurs due to the pressure gradient from the anterior part of the eye towards the posterior pole. This low level convective flow does not play any significant role for the distribution of low-molecular weight drugs. However, high-molecular weight drugs move through the vitreous as a result of bulk flow.
In order to evaluate the convective–diffusive drug transport within the eye, the fluid velocity was obtained by solving for creeping flow in a porous medium using Darcy’s Law. According to Darcy’s law, in laminar flows through porous media, the pressure drop is proportional to velocity as:
The aqueous humor is incompressible, hence:
where, \( \overrightarrow v \) is the velocity of the fluid, K h is the hydraulic conductivity of the vitreous gel, μ is the viscosity of the fluid, P is the pressure, and ρ is the density. The flow is assumed to be steady and independent of the drug concentration.
To obtain the drug distribution, the species mass conservation equation was coupled with the flow field as:
where, C is the concentration of the drug, D is the diffusion coefficient of drug in the vitreous, and q is the generation/consumption rate of the drug. It is assumed that the drug is not metabolized or degraded within the vitreous, so q was set to zero.
Boundary Conditions
There are three main tissues that bound the vitreous humor: the hyaloid membrane, lens, and RCS layer. Boundary conditions are summarized in Table VIII.
The hyaloid membrane, which separates the vitreous humor from the anterior segment of the eye, is a suspensory ligament connecting lens and ciliary processes and is composed of loosely packed noncollageneous protein. Following Balachandran and Barocas (29), a flux boundary condition has been considered to represent the loss of the drug to the anterior segment. Assuming that the aqueous humor in the posterior chamber is well mixed, the flux of the drug at the hyaloid surface is expressed as:
Where f is the flow rate of aqueous humor [3 μl/min], A is the hyaloid membrane area (1.78 cm2), and C is the concentration at the hyaloid surface.
Pressure at the hyaloid membrane is considered to be the same as that of aqueous humor, which is close to the intraocular pressure (IOP) of the eye. For a healthy eye, IOP is generally between 15 and 20 mmHg (2000–2666 Pa), whereas for a glaucomatous eye it can rise up to 40–80 mmHg (5333–10666 Pa) (30). In this study, pressure on hyaloid membrane is considered to be 15 mmHg (2000 Pa) for the normal eye.
The lens is assumed to be impermeable to both flow and the drug concentration. Therefore, at the surface of the lens a no-flux boundary condition for concentration and no slip boundary condition for flow have been applied.
The vitreous cavity is surrounded by several layers which give it mechanical support. Liquid leaving the vitreous chamber passes first through the retina, then the pigment epithelium, then through a loose capillary bed (the choroid and suprachoroid), then through the sclera, and finally through the loose episeleral tissue covering the eye. For the purpose of modelling, we have lumped the different tissues around the vitreous into one entity (RCS layer).
The boundary condition for momentum equation can be expressed by using Darcy’s law as below:
where K h is the hydraulic conductivity of RCS layer, μ is the aqueous humor viscosity, P is the pressure on the RCS layer adjacent to the vitreous, Pv is the pressure of the episcleral tissue (27) that is 9 mmHg (1200 Pa) and L is RCS thickness.
For the concentration boundary condition, both convective and diffusive transport of the drug from the vitreous to the retina has been considered. Various transport processes in RCS membrane control the movement of drug out of the vitreous chamber. The influence of all transport mechanisms out of the vitreous is incorporated in an unknown parameter which is referred to as RCS permeability, KRCS, as:
Initial Condition
To model an intravitreal bolus injection of a specific value of drug, it is assumed that the drug is injected at the center of the vitreous chamber. This assumption is based on the method of injection used in all the previous pharmacokinetic experiments. The injected drug initially has a homogenous distribution within a spherical region. These assumptions are based on the injection method which was mentioned in related papers. The initial size of the spherical region is according to the volume of injected drug. The density of the drug solution is considered to be same as that of water i.e., 1000 kg/m3. The initial normalized mass fraction of the drug is assumed to be 1 (normalized with respect to the concentration of the drug in the water base) within the domain of the drug injection site while it is 0 in rest of the vitreous. In mathematical words:
Model Parameters
The parameters required to solve the model are intraocular pressure (IOP), viscosity of the aqueous humor, hydraulic conductivity of the vitreous and RCS layer, drug diffusivity in the vitreous and RCS permeability. Of these parameters, two parameters depend on drug physicochemical properties and need to be calculated. These two parameters are drug vitreous diffusivity (D) and RCS layer permeability (KRCS). The Stokes-Einstein equation was used to estimate drug vitreous diffusivity;
where k is the Boltzman Constant (1.38 × 10−23 J/K), T is the temperature, η is the viscosity of the solvent, and r is the solute molecule radius which was calculated by:
where MW is the solute molecular weight, N is the Avogadro’s number and ρ is the density. Substituting Equation a8 into Stokes-Einstein equation gives:
Therefore, drug diffusivity is inversely proportional to the cube-root of the molecular weight. The diffusivity of fluorescein in the vitreous humor has been found experimentally by Araie et al.(5) to be 6 × 10−10 m2/s. This value was used as base and vitrous diffusivity of other drugs was calculated as below:
For estimation of KRCS, an initial guess for KRCS was made based on the mean residence time of the drug in the vitreous. Later on the optimum value of KRCS was obtained by minimizing the objective function y as described before.
The hydraulic conductivity of vitreous (Kh/μ)vit and RCS membrane (Kp) were considered to be 8.4 × 10−11 m2/Pa.s and 5 × 10−12 m/Pa.s, respectively (31).
Computer Code
CFD calculations were conducted using FLUENT software version 12.1.4 (ANSYS, Inc., Canonsburg, PA). This code is based on a control volume approach where the computational domain is divided to a number of cells and the governing equations are discretized into algebraic equations in each cell. These equations satisfy the integral conservation of the mass and the momentum over each control volume. Geometrical models and meshing were constructed using GAMBIT version 2.2.30.
Grid Independence Study
To check whether the size of grid is sufficient, grid size independence study has been done. A good grid size is one that has no influence on the results. When the grid is too fine numerical round off errors appear and when it is too course one could get truncation errors. Grid independence means that the converged solution obtained from a CFD calculation is independent of the grid density. As it is shown in Fig. 5 simulation with 604 cells and 2322 cells, creates less than 1.5% variation in the magnitude of velocity in the centreline of vitreous. This good agreement between the results from the two grid levels justifies the use of 604 cells for the simulation.
Velocity and Pressure Contour
Fig. 6 Shows the predicted fluid velocity contour within the vitreous chamber. The average velocity across the RCS surface was 4.0 × 10−9 m/s, and the average velocity in the vitreous was 7.12 × 10−9 m/s, which are consistent with earlier analysis (31).
Fig. 7 Shows the pressure distribution within the vitreous. The higher pressure specified at the hyaloid membrane (15 mmHg = 2000 Pa) drives the aqueous from the hyaloid to the outer sclera which is maintained at a venous pressure of 9 mmHg (1200 Pa). It is observed that the pressure drop across the vitreous is about 0.5 Pa (0.004 mmHg). Therefore, most of the pressure drop occurs in the RCS membrane.
APPENDIX II







Rights and permissions
About this article
Cite this article
Haghjou, N., Abdekhodaie, M.J. & Cheng, YL. Retina-Choroid-Sclera Permeability for Ophthalmic Drugs in the Vitreous to Blood Direction: Quantitative Assessment. Pharm Res 30, 41–59 (2013). https://doi.org/10.1007/s11095-012-0847-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0847-9